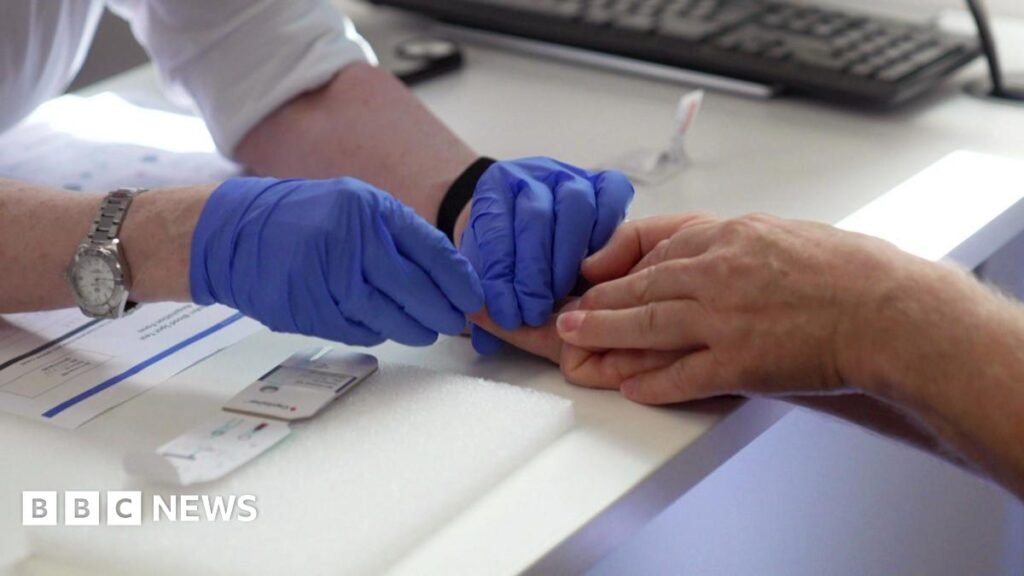
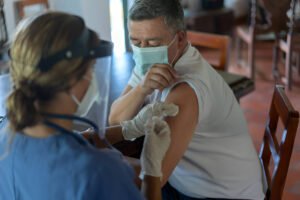
A new Alzheimer’s finger-prick test is being studied as a potential breakthrough in the early diagnosis of Alzheimer’s disease, offering a faster, cheaper and less invasive alternative to current testing methods.
The international trial involves 1,000 volunteers aged over 60 across the UK, the United States and Canada, and aims to identify blood-based biomarkers linked to Alzheimer’s.
How the Alzheimer’s Finger-Prick Test Works
The test analyses small blood samples taken from a finger prick to detect three specific proteins associated with Alzheimer’s disease. These proteins are known to be linked to changes in the brain that occur long before symptoms appear.
Dr Giovanna Lalli, director of strategy and operations at LifeArc, explained that measuring the levels of these proteins may help determine whether a person is at risk of developing Alzheimer’s.
Research shows that abnormal proteins such as amyloid and tau can build up in the brain up to 15 years before symptoms begin, making early detection critical.
Trial Participants and Study Design
Volunteers taking part in the study will also undergo current gold-standard diagnostic tests, including brain PET scans or lumbar punctures. These methods, while accurate, are expensive, time-consuming and invasive.
Currently, only a small proportion of people with Alzheimer’s are offered these tests, which often leads to delayed diagnosis.
One participant, Dr Michael Sandberg, a London GP, joined the trial after witnessing his mother’s experience with Alzheimer’s. Both his brain scan and finger-prick test results were negative, which he described as a huge relief.
Why Early Diagnosis Matters
Experts say the Alzheimer’s finger-prick test could significantly improve access to diagnosis. Prof Fiona Carragher, chief policy and research officer at the Alzheimer’s Society, highlighted that many people wait far too long for an accurate diagnosis.
With new dementia treatments emerging, early and reliable diagnosis is becoming increasingly important for patient care and treatment planning.
Potential Benefits for the NHS
A major advantage of the finger-prick test is that it could eventually be carried out at home. Samples could be posted to a laboratory for analysis without refrigeration, reducing pressure on NHS services and specialist clinics.
If proven effective, the test could be used as a screening tool for older adults, helping identify risk earlier and prioritise further assessment where needed.
Progress of the Trial
So far, 883 participants have been enrolled, with more than 360 completing all tests. The study includes people who are cognitively healthy, mildly impaired and those in the early stages of Alzheimer’s.
At least 25% of volunteers come from under-represented groups, ensuring broader representation. The trial is expected to conclude in 2028, after which researchers will analyse the full data set to determine the accuracy and reliability of the test.
The Future of Blood-Based Alzheimer’s Testing
Blood-based testing for Alzheimer’s is a rapidly advancing area of research. In 2024, US regulators approved a blood test using traditional blood draws, and further UK studies are exploring how blood tests could improve real-world diagnosis and treatment.
If successful, the Alzheimer’s finger-prick test could mark a major shift in how dementia is detected, making early diagnosis more accessible, less invasive and more widely available.
Health Research and Safety Awareness
Organisations focused on health research and prevention, such as OSHAssociation.org, play an important role in raising awareness about emerging medical technologies and their impact on public health. Access to reliable health information supports informed decision-making and encourages proactive approaches to long-term wellbeing.
🔹 FAQs
What is the Alzheimer’s finger-prick test?
It is a blood test that uses a small finger-prick sample to detect biomarkers linked to Alzheimer’s disease.
How is it different from current tests?
Unlike brain scans or lumbar punctures, it is less invasive, quicker and potentially cheaper.
Can the test be done at home?
Researchers hope it could eventually be carried out at home, with samples sent to a lab for analysis.
When will the study finish?
The trial is expected to complete in 2028 after analysing results from all participants.